Contact us today:

(847) 934-4500
tdaro@bernardandcompany.com

Contact us today:
(847) 934-4500
tdaro@bernardandcompany.com

Working together with RJG at the Medical Devices conference, the goal is to provide attendees with a demonstration on how to utilize currently available technology to their best advantage. Reducing time to market with higher quality and repeatable molding processes are key to the future success of injection molding professionals and OEM’s.
RJG and SIGMA will take you through the critical steps from product design to production with best practices for successful, profitable molding. Develop and merge the part design, the polymer, the mold, and the process in a virtual production environment where all of the critical aspects related to profitable part quality can be evaluated and optimized before the actual mold is ever built.
This is an actual workshop with worksheets and exercises that can be used to develop improved communications within your work environment.
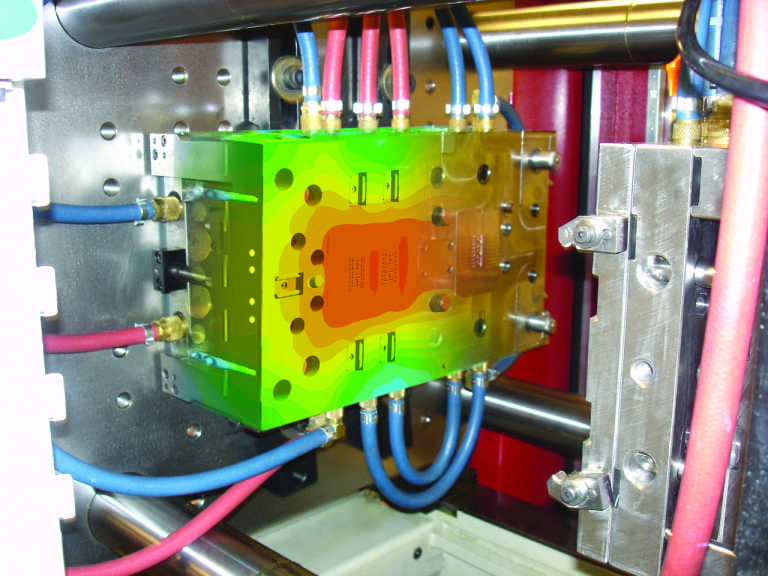
Should the mold insert be P20, H13, or a Cu based alloy?
Where are the most critical areas for cooling?
What will the cycle time be?
Is the distortion related to fiber orientation or temperature?
Can it be controlled with packing?
Will it be pressure limited when the viscosity shifts?
How big is the process window?
Can the process be maintained?
Where do we need sensors?
How to contain parts produces outside of the process window
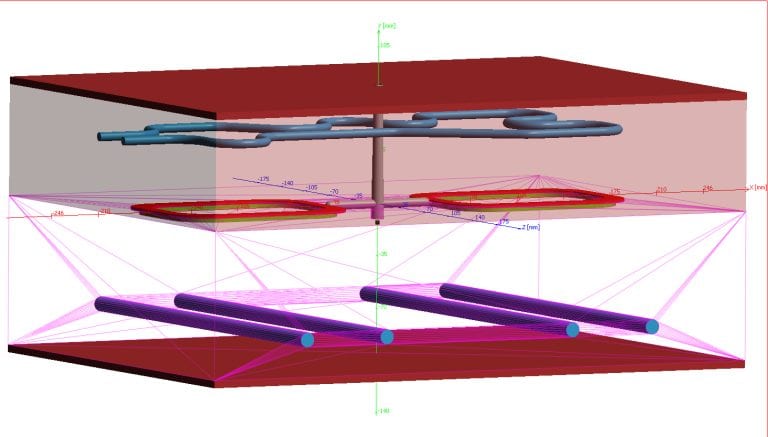
If the mold is already built and the part dimensions from the quoted 30s cycle are out of spec, what are you going to do about it, other than lose money…..? There are more profitable ways of doing things.
For more information, contact:
Matt Proske
Vice President
SIGMA Plastic Services, Inc.
10 N. Martingale Road, Suite 425
Schaumburg, IL 60173
Phone 847-558-5602
Email contact@3dsigma.com
Web www.3dsigma.com