Contact us today:

(847) 934-4500
tdaro@bernardandcompany.com

Contact us today:
(847) 934-4500
tdaro@bernardandcompany.com
 Banner Medical is committed to a uniquely ambitious approach to quality assurance – developed specifically for the evolving, critical needs of the medical device industry. To date, no other medical device materials supplier has achieved a greater degree of sophistication or engaged in a more data-intensive approach to developing and bullet-proofing processes. Banner believes this investment achieves multiple payoffs – in relationship-building with customers, in risk mitigation, and in long-term success for all.
Banner Medical is committed to a uniquely ambitious approach to quality assurance – developed specifically for the evolving, critical needs of the medical device industry. To date, no other medical device materials supplier has achieved a greater degree of sophistication or engaged in a more data-intensive approach to developing and bullet-proofing processes. Banner believes this investment achieves multiple payoffs – in relationship-building with customers, in risk mitigation, and in long-term success for all.
The background of Banner’s industry-leading quality assurance program and supply chain management solution includes many and varied factors. Each individually could provide justification for a major initiative on the part of a responsible, forward-thinking supplier. Collectively, these reasons make an inarguable case for new, stronger management of the supply chain.
Let’s review the reasons why Banner has so vigorously engaged in the industry’s leading quality assurance program.
Reason #1: Because the supply chain needs help
The medical device industry has witnessed a series of costly and dangerous failures resulting from the dilution of device manufacturer standards down the supply chain. The buyer of materials must indeed beware of superficial quality measures and undocumented changes.
An approach that emphasizes prevention represents a viable solution. By investing in supplier relationships premised on stringent purchasing controls, the device manufacturer considerably diminishes the likelihood of a hugely expensive failure and recall with liability. The 1:10:100 rule holds that a dollar spent on prevention of a quality problem forestalls the expenditure of $10 on correction or $100 to rectify a failure.
In Japan and Germany, single sourcing is common and relationships can be decades long; suppliers are considered an extension of the customer company’s operation. American firms have historically not taken this approach. So, American device manufacturers generally work with approved supplier lists that have grown large and consequently difficult to manage.
In the past, device manufacturers commonly worked with multiple suppliers for the same material, to minimize the possibility of a supply shortage or to bring down material price through competition. Today, short-term cost savings are used to justify the multiple-supplier, purchase-order-to-purchase order method. Such near-term savings, however, come with a high potential price: failures that can literally put a device manufacturer out of business. In recent years, thoughtful device manufacturers have concluded that they possess minimal leverage in applying controls to metal melt sources. More than 95% of a typical melt source’s business is outside the medical industry; the melt sources have little incentive to change. Furthermore, device manufacturers often do not buy directly from the melt sources and lacked direct access and contact.
In dealing with suppliers, it is critical that a device manufacturer know how the supplier’s systems have evolved, as did Banner’s, in response to medical device industry needs. Additionally, procedures used to determine acceptability of suppliers must be unambiguous. The ASL should itemize specific products and processes for which the supplier is approved, not just the name of the company. A supplier that has been approved for one product or process should never be assumed to be approved for everything.
Purchasing controls can take many directions in the effort to achieve greater stringency. One promising approach is for device manufacturers to focus on building strategic partnerships with suppliers with the demonstrated capability to provide acceptable product.
Reason #2: Because we’ve got the data
In a time frame coinciding with several high-profile and catastrophic materials problems in the medical device industry, Banner proactively validated all its medical equipment and processes (IQ, OQ & PQ) per FDA protocols. Furthermore, we developed proprietary and particularly stringent bullet-proofing systems to ensure risk mitigation.
Banner designed experiments to determine “worst case” scenarios and effects of processing parameters on the finished metals regarding mechanical properties and surface conditions for static and dynamic or high stress applications. Selection criteria for implant and device grades metals were based on 1) difficulty for machining, i.e. chemistry, 2) difficulty to straightening, i.e. very high mechanical properties, and 3) best commonly used coolants ~ Straight Sulfurized oils vs. water soluble coolants.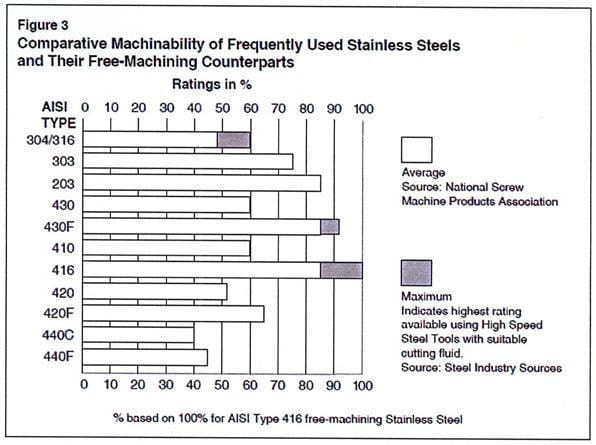
We determined that softer material such as aluminum, brass, and carbon steel shrink as much as 0.002” on several passes during straightening; meanwhile stainless, CCM and titanium had minimum deflection. Grades such as Inconel, CCM, 440, 304, and Titanium proved very difficult to turn or grind. The experiment selected 316, Ti, 17-4A, 17-4H900, 440A and CCM grades based on their status of “most difficult” to machine and because of their extensive use in medical devices and implants (SS, CCM and Ti families). Banner chose minimum and maximum sizes of 0.0250” and 1.000” (based on volume/history) on existing equipment.
For a worst-case scenario on straightening, grinding, and turning processes, bars were processed outside validated parameters. Then the supplier removed 125 percent of the routing parameter; for example, if the router states 0.010” removal, Banner tried to remove 0.0125” per grinding pass. Banner also ground samples down to 15 percent of the original diameter by volume. Samples were taken for analysis on surface condition, mechanical properties, and straightness (TIR).
Extensive and detailed protocols determined the effectiveness of equipment function, operations and processes, outputs and revalidation criteria. Hundreds of samples were sent to a 3rd party A2LA and NADCAP (Exova) accredited laboratory for analysis. Multiple samples for each scenario were run and analyzed for repeatability and reproducibility.
The tests concluded that, operating within validated procedures and processes, Banner could produce material that was compliant in terms of mechanical properties without adverse effect on the raw material. Removing 85 percent volume of stock and straightening 20 times has no negative impact on physical properties and surface conditions, as long as validated processing parameters are followed. Operating outside of the safe zone of validated protocols, processes, and parameters, Banner found adverse impacts on final produced materials.
|
Titanium Eli Annealed ASTM F136 Verified by 3rd Party Lab. (Exova) |
||||||||
|
Sample # |
Start Size |
Test Size |
0.20%YS |
UTS |
%EL |
%RA |
Cracks observed at 400x |
|
|
1 |
Ø .750″ |
Ø .251″ |
137,000 |
145,500 |
17.0 |
50.8 |
No |
|
|
2 |
Ø .750″ |
Ø .252″ |
142,000 |
149,200 |
17.0 |
51.8 |
No |
|
|
3 |
Ø .750″ |
Ø .253″ |
137,200 |
147,000 |
18.0 |
50.5 |
Yes |
|
|
4 |
Ø .750″ |
Ø .254″ |
139,200 |
148,200 |
17.0 |
48.7 |
Yes |
|
| Sample # | ||||||||
|
1 |
Material tested as received from mill | |||||||
|
2 |
Material tested as received from mill | |||||||
|
3 |
Processed outside validated parameters | |||||||
|
4 |
Processed outside validated parameters | |||||||
|
3 Point Fatigue Test Cycle Counts |
||||||||
|
Sample # |
Start Size |
Test Size |
Frequency |
Min |
Max |
~ Min |
~ Max |
Cycles to Failure |
|
1 |
Ø .750″ |
Ø .750″ |
15 |
-500 |
-5,000 |
-0.020 |
-0.105 |
16,823 |
|
2 |
Ø .750″ |
Ø .750″ |
15 |
-500 |
-5,000 |
-0.020 |
-0.105 |
15,334 |
|
3 |
Ø .750″ |
Ø .750″ |
15 |
-500 |
-5,000 |
-0.020 |
-0.105 |
13,686 |
|
4 |
Ø .750″ |
Ø .750″ |
15 |
-500 |
-5,000 |
-0.025 |
-0.120 |
4,931 |
|
5 |
Ø .750″ |
Ø .750″ |
15 |
-500 |
-5,000 |
-0.025 |
-0.120 |
3,012 |
|
6 |
Ø .750″ |
Ø .750″ |
15 |
-500 |
-5,000 |
-0.025 |
-0.120 |
3,314 |
| Sample # | ||||||||
|
1 |
Normal straighten and grind using validated processes | |||||||
|
2 |
Normal straighten and grind using validated processes | |||||||
|
3 |
Normal straighten and grind using validated processes | |||||||
|
4 |
Processed outside validated parameters | |||||||
|
5 |
Processed outside validated parameters | |||||||
|
6 |
Processed outside validated parameters | |||||||
For example, per the test performed on implantable grade Titanium ELI ASTM F136, 0.750” diameter bar results showed that excessive straightening could induce surface cracks. Although there was no significant change in the physical properties before and after the test, under dynamic loading (Fatigue Test), the Fatigue cycles were reduced by a factor of 5 when operating outside of highly controlled and proven processes. Application life under validated processes averaged about 15,000 cycles but failure occurred within 20 percent of that application life, at about 3,500 cycles, with processes outside these parameters.
For Banner, an 18-month investment in design of experiment and collection of substantial data and documentation proved most valuable. The findings provided incontrovertible evidence of the need for validated procedures and processes. For Banner customers, the data offer an assurance of quality.
Reason #3: Because the FDA says so
The supply chain stands to receive unprecedented FDA scrutiny in today’s medical device industry. For the FDA, supplier controls represent a top Quality Hot Topic. Specifically, raw materials are increasingly a cause for concern. More than one-third of all 2010 Production and Process Controls (P&PC) subsystem warning letter cites are associated with regulation of material purchasing controls and acceptance activities (Section 820.50 and 820.80). The FDA continues to add dozens of new field inspectors and contracts with yet more inspectors to increase the agency’s capacity to review purchasing controls and acceptance activities, as well as other quality system matters. This activity included the hiring of more than 700 inspectors to ensure adequate, timely inspections overseas. The medical device industry cannot afford to pay less attention to quality topics than the FDA.
 Recent history has generated considerable concern about the effectiveness of controls through the layers of suppliers to the device industry. Kimberly Trautman, Medical Device Quality Systems/GMP Expert, Office of Compliance, CDRH, FDA suggests that the industry should consider when, not if, additional events will occur. The FDA’s position is that device manufacturers must be accountable for the identification and mitigation of risk in all tiers of their supply chains. The FDA has acknowledged that, as the medical device industry continues to grow worldwide, the agency’s oversight ability has become strained despite added resources. FDA inspections and authority often extend only to the finished device manufacturer, yet manufacturing of materials, critical components and entire devices is often outsourced; increasingly such outsourcing goes overseas. In response to these realities, the FDA puts its efforts into controlling the entities over which it has jurisdiction: the finished device manufacturer. In turn, the FDA demands that device manufacturers ensure selection of “only those suppliers, contractors, and consultants who have the capability to provide quality products and services.”[1] The finished device manufacturer is ultimately the party legally responsible for compliance with the Quality System regulation and for assuring the safety and effectiveness of the finished device, a point once again emphasized in an August, 2011 FDA Supplier Quality Management Congress presentation by Trautman.
Recent history has generated considerable concern about the effectiveness of controls through the layers of suppliers to the device industry. Kimberly Trautman, Medical Device Quality Systems/GMP Expert, Office of Compliance, CDRH, FDA suggests that the industry should consider when, not if, additional events will occur. The FDA’s position is that device manufacturers must be accountable for the identification and mitigation of risk in all tiers of their supply chains. The FDA has acknowledged that, as the medical device industry continues to grow worldwide, the agency’s oversight ability has become strained despite added resources. FDA inspections and authority often extend only to the finished device manufacturer, yet manufacturing of materials, critical components and entire devices is often outsourced; increasingly such outsourcing goes overseas. In response to these realities, the FDA puts its efforts into controlling the entities over which it has jurisdiction: the finished device manufacturer. In turn, the FDA demands that device manufacturers ensure selection of “only those suppliers, contractors, and consultants who have the capability to provide quality products and services.”[1] The finished device manufacturer is ultimately the party legally responsible for compliance with the Quality System regulation and for assuring the safety and effectiveness of the finished device, a point once again emphasized in an August, 2011 FDA Supplier Quality Management Congress presentation by Trautman.
Working within the comprehensive parameters set by the FDA, the medical device industry needs to manage its supply chain with unprecedented vigilance. Device manufacturers must take responsibility for the condition and quality of items purchased. It is imperative to identify and mitigate risks in the supply chain. Purchase decisions for devices, raw materials and services must be made with consideration to risk-based principles. Poor purchasing decisions lead to circumstances where companies will not meet regulatory or quality requirements, and will be subject to damage to their reputation and potential litigation. Conversely, purchasing decisions made with attention to the unique risks and needs of the medical device supply chain are imperative.
A strategic change in action
Banner has already employed its new approach and controls in a relationship with an important customer, with mutual benefits and recognized success. The device manufacturer revised specifications to require melt source and melt process validation, independent test lab verification, and an approved supplier list of melt sources. Every raw material lot was subject to certificate verification through an inspection plan. Banner committed to fulfilling all the new requirements, and additionally offered to have the material pretested by a major OEM-approved lab. This meant the device manufacturer could end its practice of cutting and sending out test samples from their received material, a process that had delayed stocking of the material for two weeks.
The device manufacturer challenged Banner to “bullet-proof” material controls to prevent mixing or sending incorrect material. In response, Banner offered its unique statistical process control system tailored to the device industry. Statistical process control can be employed as a validation activity that helps deliver understanding of the impact of change. In this instance, customized IT / MRP System controls automate error proofing of certification, verification of mechanicals and chemistry, restrictions, and more. Customized software that uses control plans can include detailed requirements for various “inputs,” for example, inspection plans and test results. The computer logic Passes or Fails each step of product realization. When certain criteria are met, security is triggered to escalate the issue to appropriate personnel in the organization. In the supply chain, particularly important considerations would include restrictions and exceptions. A “No China Titanium” restriction offers one example. In another example, information could be input to specify a supplier’s qualification only for heat-treating and not for sterilization. When the system controls all this information, only data that meet established criteria will automatically flow through. The automation minimizes the need for manual intervention and accompanying human error.
This system controls what is supplied and where it is sourced, literally stopping the process if the wrong combination is entered, constituting what is essentially an approved supplier list imbedded in the supplier’s IT system. Any requirement for an outside lab test is included in the customer profile. The program also has a function that requires entry of actual material test results. If any specification requirement is unmet, the material is locked down and cannot be shipped without overriding the system. As of late 2011, Banner’s customized Quality Management IT System has ‘Patent Pending’ status with the U.S. Patent and Trademark Office.
Additionally, Advanced Product Quality Planning, a process successfully employed in other industries including automotive and aerospace, was incorporated into the partners’ business process. Cross-functional teams mapped the administrative process step by step, developed a Failure Mode Effects Analysis (FMEA) ranking scheme, and employed several other quality tools. Upon completion of the extensive analysis, the supplier revised and developed improved work instructions and forms. This partnership innovated again by applying quality controls to the administrative function.
Banner and its customer identified several additional factors that contributed to the success of the strategic partnership. Both firms entered into the partnership with full management support. Both organizational cultures valued teamwork and cooperation. Both parties committed to short- and long-term goals and structured contractual agreements and business processes accordingly. The companies in the strategic partnership recognized and adeptly worked toward their common best interest.
Banner believes that long-term strategic partnership minimizes total cost and increases competitiveness for both partners. For Banner, guaranteed business fosters the creation of long-term plans for growth and resource requirements. Banner’s device manufacturer customers benefit from greater speed to market as vendor-managed inventory (VMI) reduces lead times. Both parties save time and money as administrative costs decrease and shipments are consolidated. Most importantly, device manufacturers benefit from reduced risk when medical-grade material is a core competency of the supplier.
Banner is a metals supplier whose interests and systems align with the medical device industry and with the FDA expectations by which the industry must abide. Banner has invested in the development of stringent processes and tools to address the specialized needs of device manufacturers. A strategic partnership offers greater levels of control, ensures timely delivery, and maximizes regulatory compliance. In the longer term, these supply chain management approaches help support the FDA mandate for TPLC accountability and establish a process for efficient product development, increasing the speed at which Banner’s customers can bring successful products to market while managing risk.
Banner Medical intends to be the metals supplier who shares the priorities of the device industry and possesses the capabilities to ensure timely delivery with no adverse effects.
[1] Preamble to the 1996 QS Regulation, Comment #106